Global Footprint
Our Facilities
Adare has a global manufacturing footprint, capable of serving markets throughout the world. From our state-of-the-art facilities in the US and Europe, we can provide development-scale through commercial-scale production. Adare offers a continuum of highly specialized manufacturing services and capabilities to meet your oral solid dose needs. View a virtual tour of our Vandalia site.
-

Vandalia, Ohio
Key Facts:
179,000 sq ft facility (16.6k sq m)
—
R&D center
—
Commercial scale manufacturing
—
Organic solvent capabilities
—
Manufacturing License for DEA Schedules II, 2N, III, IV, V -

San Giuliano (Milan), Italy
Key Facts:
93,000 sq ft facility (8.6k sq m)
—
Commercial scale manufacturing
—
Focused on controlled release technologies
—
Organic solvent capabilities
—
Tablet & capsule filling -

Pessano (Milan), Italy
Key Facts:
220,000 sq ft facility (20.4k sq m)
—
Expanded R&D capabilities
—
Clinical & commercial scale manufacturing
—
Organic solvent capabilities
—
Controlled substances -

Lenexa, Kansas
Key Facts:
5,616 sq ft facility (465 sq m)
—
R&D center
—
Non-GMP development scale
—
Long-acting injectables -

Aurora, Illinois
Key Facts:
33,000 sq ft facility (3k sq m)
—
R&D center
—
Commercial scale manufacturing
—
Fluid bed technology center
—
Manufacturing License for DEA Schedules II, 2N, III, 3N, IV, V, L1 -

Philadelphia, PA (Orthodox)
Key Facts:
128,000 sq ft facility (12k sq m)
—
R&D center
—
Commercial scale manufacturing
—
High potency manufacturing site
—
Manufacturing & Packaging Licenses for DEA Schedules II, 2N, III, 3N, IV, L1 -

Philadelphia, PA (Dungan)
Key Facts:
175,000 sq ft facility (16.2k sq m)
—
Packaging & Warehousing
—
4 packaging lines & 1 powder filling line
—
Packaging of DEA Schedules 2N, III, 3N, IV -
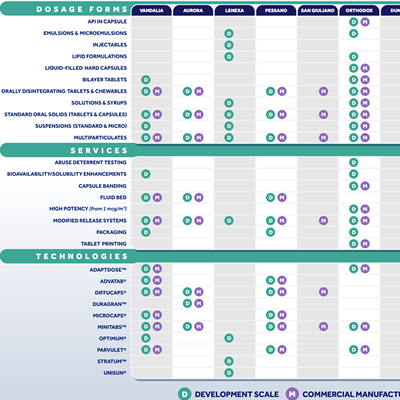
Site Capablities
From novel dosage forms, innovative technologies, and unique CDMO services, our network of state-of-the art facilities provide you with a wide range of capabilities that ensure your next project is a success.
To find out more about what each site in our global network offers, click the chart above.
